Selection of patients
Referring hospital: haematologist and oncologist
- Assessing whether the patient is eligible and suitable for CAR T treatment with YESCARTA® in DLBCL
- Informing the patient about CAR T treatment with YESCARTA®
- and managing expectations before referral
Medical and clinical responsibility
Assessing whether the patient is eligible and suitable for CAR T treatment with YESCARTA®
Referring hospital: haematologist and oncologist
YESCARTA® (axicabtagene ciloleucel) is indicated for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL), after two or more lines of systemic therapy.¹
Yescarta is indicated for the treatment of adult patients with diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBL) that relapses within 12 months from completion of, or is refractory to, first-line chemoimmunotherapy.¹
YESCARTA® is an engineered autologous T-cell immunotherapy product. It involves a patient’s T cells being harvested, genetically modified in the lab to attack cells carrying CD19, and infused back into the same patient.¹
For more information on how YESCARTA® works as well as its efficacy and safety, see YESCARTA® product articles.
The NT Council recommends the use of YESCARTA(R) in both 3L and 2L DLBCL in accordance with approved EMA indications.2,7
Here, we address suitability of patients for therapy based on clinical eligibility only. See the Referral part of the treatment journey for more information on patient access in Sweden.
Additional eligibility considerations based on the protocol for the Phase 2 clinical trial of YESCARTA® in large B cell lymphoma (ZUMA-1) are shown in the table.
Consideration should also be given to prior therapies the patient has received and any ongoing toxicities, as these may affect the collection of adequate T cells required to make YESCARTA®. 1,5,6
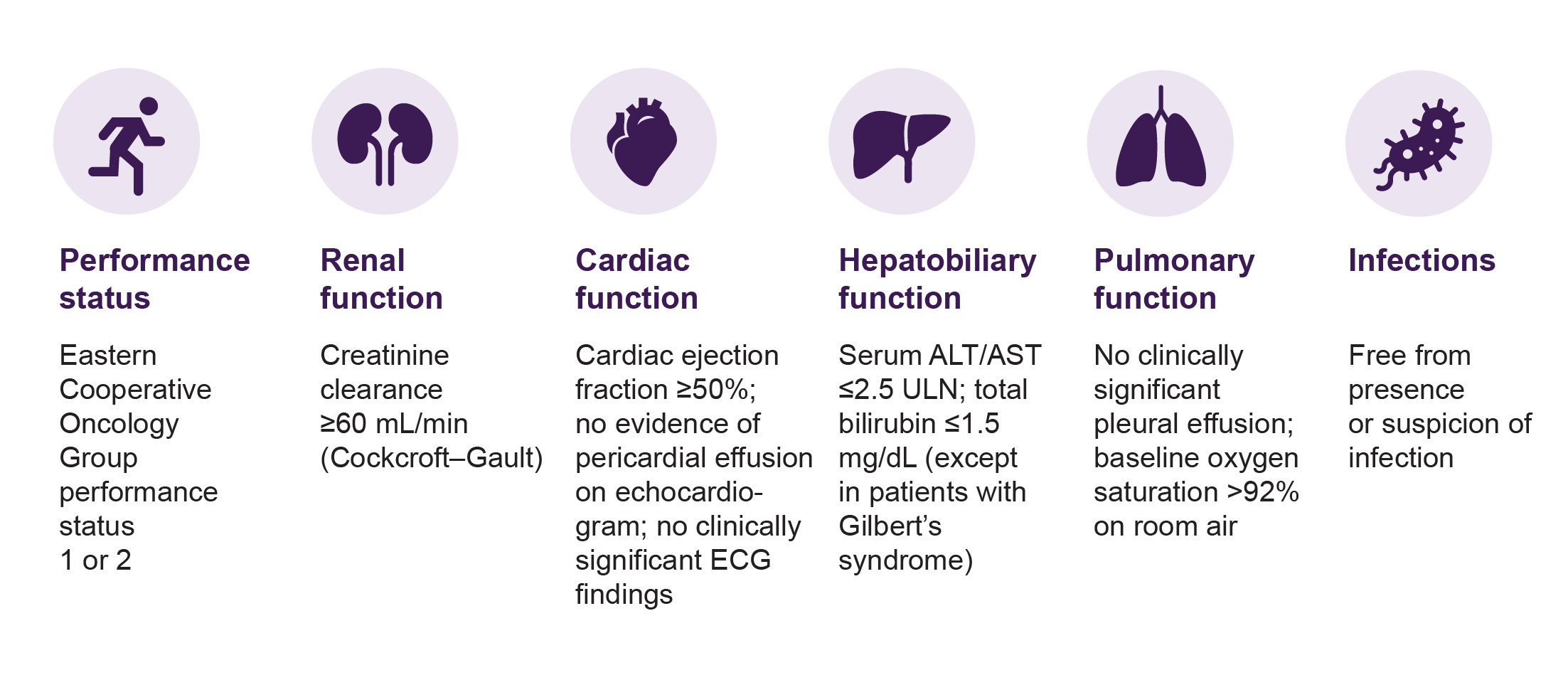
TABLE: Eligibility for YESCARTA® based on performance status, organ function, and infection³ Information based on the protocol for: Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44. ALT = alanine aminotransferase; AST = aspartate transaminase; ULN = upper limited of normal.
Based on inclusion criteria and international experience, the following guidelines for treatment with Yescarta have been prepared by the Swedish RCC-CAR T group. For the most part, these comply with the inclusion criteria in the ZUMA-1 study. A guideline is that the general performance status and organ function together must correspond to what is normally required for an autologous transplant. CAR T cell therapy should only be administered by qualified centers and preceded by national MDK. 4
Informing the patient about CAR T treatment with YESCARTA® and managing expectations before referral
Haematologist or oncologist
Although potentially eligible patients will be treatment-experienced, the CAR T treatment process is distinct to that of other lymphoma treatments the patient will have received. As well as explaining how the treatment works and the potential benefits and risks, the referring physician should ensure that the patient:
- appreciates that there are multiple steps in the treatment process¹
- understands that there will be a wait of about 4 weeks between the collection of their T cells and administration of YESCARTA®¹
- accepts that there is a high risk of potentially serious side effects including cytokine release syndrome (CRS) and neurological adverse reactions¹
- is able to meet the requirements of therapy, including staying near the treating hospital for 4 weeks after infusion¹
- is amenable to long-term follow up³
The patient will receive further information and support at the treating hospital to help them to decide whether they wish to proceed with YESCARTA® treatment. However, early discussion supports patients in considering the therapy and having realistic expectations.
References
- Yescarta SmPC
- NT-council recommendation for Yescarta in 3L DLBCL at https://www.xn--ntrdet-kua.se/nationelltinforandeavlakemedel/produktinfo/yescartaaxicabtageneciloleucel.4.737fc4451643b8af77bdb09.html. Accessed February 2023.
- Protocol for Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44.
- Regionala cancercentrum i samverkan. Aggressiva B-cellslymfom. Nationellt vårdprogram, 2023-01-25 Version: 6.0 https://kunskapsbanken.cancercentrum.se/diagnoser/aggressiva-b-cellslymfom/vardprogram/. Accessed February 2023
- Jain T, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 2019;25:2305-21.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): B-cell lymphomas, Version 2.2021. 16 February 2021.
- NT-council recommendation for Yescarta in 2L DLBCL at https://samverkanlakemedel.se/download/18.25cdd0fd18e65a1a5d02913e/1705051242144/Yescarta%202L%20DLBCL%202024-01-12.pdf Accessed September 2024.
SE-YES-0018 | 09/2024
Focus on the patient
Common patient questions
Anticipating and addressing the needs and concerns of patients
Patients may have concerns and misconceptions about genetically modified therapies and immunotherapies, so it is important to explain how YESCARTA® works and the safety measures that will be in place.
Patients’ questions may include:
- Why do you think CAR T therapy is an option for me?
- What are my alternative options?
- Are genetically modified cells safe to receive?
- What are the chances that I will achieve remission with CAR T therapy?
- What difference might CAR T treatment make to my life expectancy?
- Could anything go wrong with my treatment?
- What if I change my mind about having YESCARTA®?
Information for patients
If you would like patient-friendly information on YESCARTA® to help you to explain this treatment to patients, please Contact Us to make a request.
In addition, you may wish to provide to your patients the contact details of trusted patient groups for more information and support on CAR T therapy.*
*Kite does not endorse or make any assurances regarding the accuracy of information on CAR T therapy provided by third party organizations.

