Post-leukapheresis
Treating hospital: haematologist and oncologist
- Consideration of bridging therapy for the patient
- Processing of the T cells and engineering of CAR T cells (YESCARTA®/TECARTUS®) by Kite
- Coordination of the delivery date
Medical and clinical responsibility
Consideration of bridging therapy for the patient
Treating hospital: haematologist, oncologist, and referring physician
The Kite manufacturing process supports a turnaround time from leukapheresis to return of the patient-specific YESCARTA®/TECARTUS® product of around 4 weeks.¹ During this time, the patient may be under the care of the treating hospital or referring physician, depending on the logistics for the patient. In either scenario, the CAR T treatment team and referring physician, together with the patient, collaborate closely to optimally manage the patient’s symptoms and disease while YESCARTA®/TECARTUS® is manufactured.²
Careful disease management at this stage is critical, as by nature of their eligibility for CAR T therapy, patients have active, more aggressive disease.² Disease progression and related adverse events can prevent the administration of YESCARTA®/TECARTUS® and should be avoided where possible.² Treatment during the waiting period – known as ‘bridging therapy’ – is an option to reduce risk of progression.² That said, patients with lower disease burden or slower tumour growth who can be closely monitored during the wait period may not require bridging therapy.² In a real-world study in the US, 53% (146/276) of patients received bridging therapy between leukapheresis and conditioning therapy.3
Choices of bridging therapy may include, systemic chemotherapy, targeted agents (such as lenalidomide or ibrutinib), palliative radiation therapy, and cortico- steroids.2 4 * Considerations when planning bridging therapy include:²
- patient tolerance and response to prior therapies
- location and volume of disease
- comorbidities and pre-existing organ dysfunction
- performance status
It is important that use of bridging therapy does not jeopardize the effectiveness of upcoming YESCARTA®/TECARTUS® therapy. Highly toxic chemotherapy regimens that could cause side effects that delay or preclude YESCARTA®/TECARTUS® infusion should be avoided². A washout period (from a day to weeks, depending on the bridging therapy used) may be required prior to lymphodepletion.²
*Bridging therapy with systemic chemotherapy, immunotherapy, targeted agents, radiation, or high-dose corticosteroids between leukapheresis and YESCARTA®/TECARTUS® infusion was not permitted during the ZUMA-1 Phase 2 trial of YESCARTA®/TECARTUS® in refractory large B-cell lymphoma except if needed to treat disease progression.5,6 Pharmacologic doses of corticosteroids and other immunosuppressive drugs were permitted where medically indicated to treat a new toxicity.6
Processing of the T cells and engineering of CAR T cells (YESCARTA®/TECARTUS®) by Kite
Kite manufacturing facilities in the Netherlands
Despite great variation in apheresis material from patients, Kite’s manufacturing process is over 99% reliable: in a clinical trial, YESCARTA® CAR T cells were successfully produced for 110 of 111 patients.5,7
Currently the real-world data from Europe shows 96% manufacturing success rate for YESCARTA®.10
Once received at Kite’s EU manufacturing facility in the Netherlands, processing of the patient’s apheresis product begins. Through Kite’s centrifugation process, unwanted cells are removed and the T-cells are selected.7,8
In the next step of the process, the patient’s T cells are activated, genetically engineered, and expanded over 9 days. Key steps in this process are:1,7,8,9
- The T cells are thawed, activated (using anti-CD3 antibody and recombinant human interleukin-2), and cultured. Activation is needed for the later stages of expansion and transduction.
- The T cells are transferred into a culture bag coated in retronectin to optimize transduction.
- The retroviral vector is added to the bag and transduction occurs (the introduction of the chimeric antigen receptor gene into the patient’s T cells).
- The cells are expanded to achieve the clinical dose of viable cells required (2 x 106 viable CAR T cells/kg).
- The cells are harvested and the final formulation is made and cryopreserved.
Following quality control checks, the patient-specific YESCARTA®/TECARTUS® product is shipped in a liquid nitrogen shipper to the treating hospital. 7
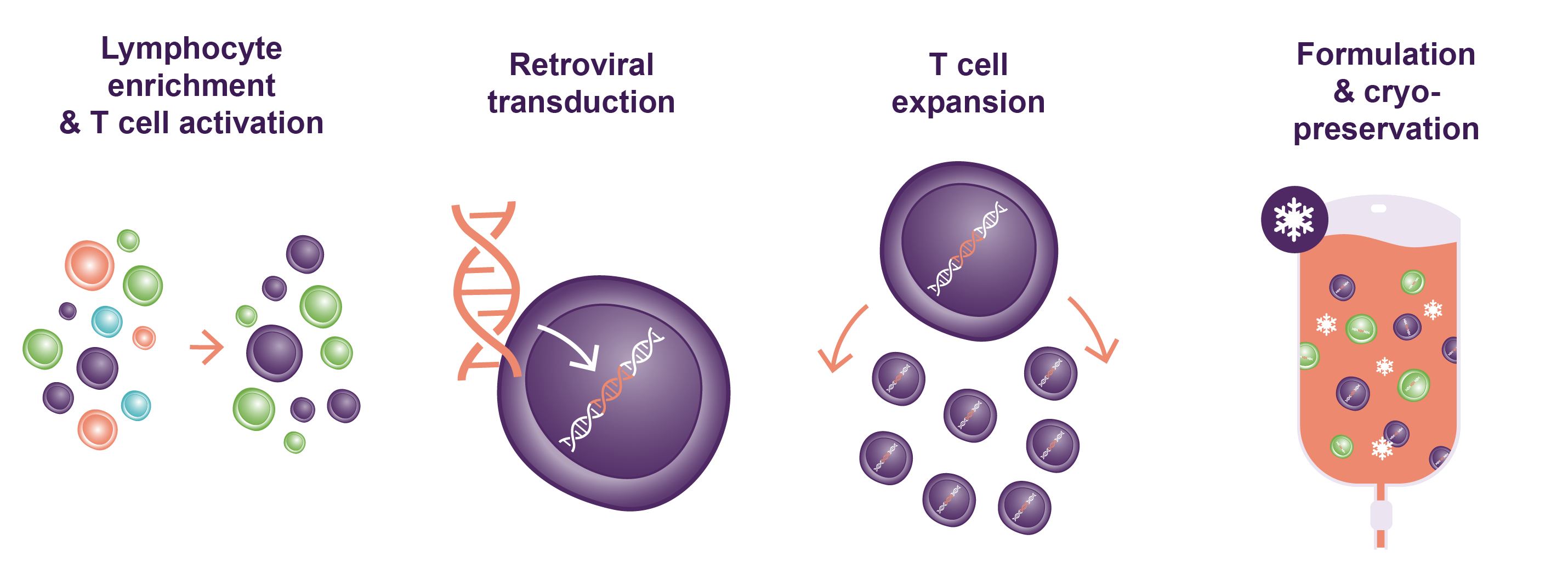
FIGURE: Visual summary of key steps in the manufacture of YESCARTA®/TECARTUS®. Adapted from Iyer RK, et al. Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges. Frontiers Med. 5:150. 8
Coordination of the delivery date
Treating hospital: haematologist, oncologist, and CAR T cell coordinator
Throughout the manufacturing process, the patient’s CAR T treatment team regularly tracks order status through the Kite Konnect® online portal and email notifications. This allows the team to schedule the patient’s conditioning treatment and YESCARTA®/TECARTUS® administration accordingly and instigate any washout periods necessary after bridging therapy.
Once the YESCARTA®/TECARTUS® product has passed quality control checks, the Kite case manager schedules the delivery date of the final product with the CAR T treatment team.
References
- Yescarta SmPC
- Jain T, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 2019;25:2305-21.
- Jain T, et al. Characteristics and outcomes of patients receiving bridging therapy while awaiting manufacture of standard care axicabtagene ciloleucel CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B cell lymphoma: results from the US Lymphoma CAR T Consortium. Blood 2019;134(Suppl 1):245.
- Riedell PA, et al. Safety and efficacy of axicabtagene ciloleucel in refractory large B-cell lymphomas. Ther Adv Hematol 2020;11:1-12.
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44.
- Protocol for Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44.
- Better M, et al. Overcoming the challenges for engineering autologous T cell therapies. Cell Gene Therapy Insights 2018;4:174-86.
- Iyer RK, et al. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges. Front Med 2018;5:150.
- Wang X, et al. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Molecular Therapy — Oncolytics 2016;3:16015.
- Kite Pharma Data on File. February 2023.
SE-YES-0022 | 09/2024
Focus on the patient
Common patient questions
Anticipating and addressing the needs and concerns of patients
The wait during CAR T cell manufacture can be a source of fear and frustration for patients. Reassure each patient about how their disease will be monitored and treated during this time. However, be open in acknowledging any risks of progression or adverse events depending on each patient’s prognosis and any bridging therapy used.
Patients’ questions may include:
- When will my CAR T cells be ready?
- What if there are not sufficient healthy T cells to make my therapy?
- Does the manufacturing process ever go wrong?
- How can I stay as healthy as possible to be ready for my CAR T treatment?
- Might my lymphoma worsen during the wait for YESCARTA®/TECARTUS®?

Information for patients
If you would like patient-friendly information on YESCARTA®/TECARTUS® to help you to explain this treatment to patients, please Contact Us to make a request.
In addition, you may wish to provide to your patients the contact details of trusted patient groups for more information and support on CAR T therapy.*
*Kite does not endorse or make any assurances regarding the accuracy of information on CAR T therapy provided by third party organizations.
