Monitoring at hospital
Treating hospital: haematologist, oncologist, and nurse
- Daily monitoring to Day 7
- Reporting adverse events
- Instructing patients, relatives, and carers on adverse events
Medical and clinical responsibility
Daily monitoring to Day 7
Treating hospital: haematologist, oncologist, and nurse
The CAR T treatment team monitors the patient daily at the treating hospital for the first 7 days after YESCARTA®/TECARTUS® infusion for signs and symptoms of cytokine release syndrome (CRS), neurological adverse events, and other toxicities.1 During this time, patients may remain in hospital or should be ready to be admitted at the first signs or symptoms of CRS or neurological events.1
Although CRS and neurological adverse events are very common following YESCARTA®/TECARTUS® infusion, 98% of patients recovered in a clinical trial.1,2
Should the patient develop CRS and/or neurological adverse events, the treating physician is guided by algorithms of how to manage the patient based on severity.1 Management includes monitoring for vital signs in patients with grade ≥2 CRS and/or neurological toxicities through to ICU support for severe or life-threatening cases.1 Patients with grade ≥2 CRS are given tocilizumab every 8 hours up to a maximum of 4 doses.1 Corticosteroids are given based on CRS or neurological toxicity grade and patient response.1
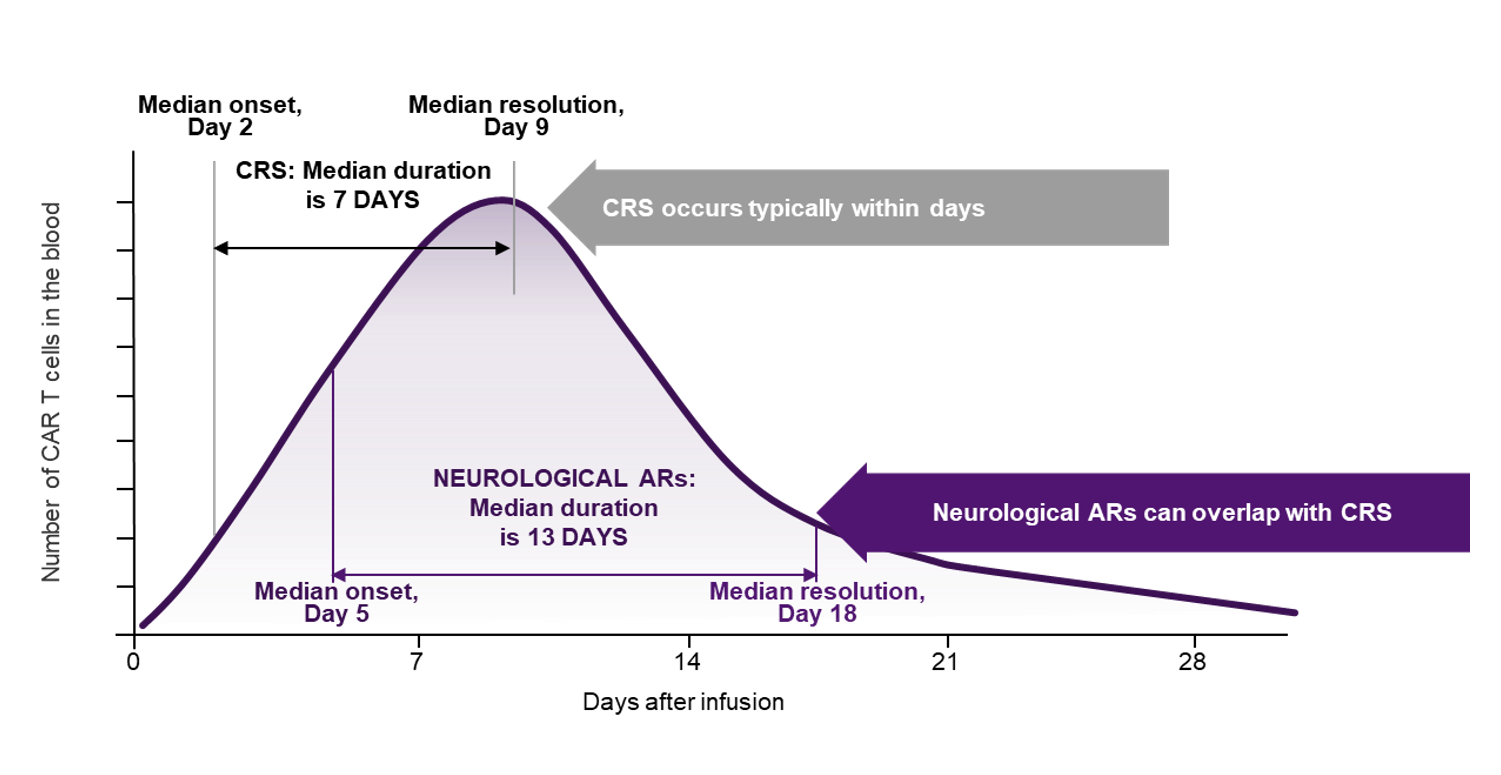
FIGURE: Median onset and duration of CRS and neurological adverse reactions (ARs) following YESCARTA®/TECARTUS® infusion. Based on information in the YESCARTA®/TECARTUS® summary of product characteristics¹
The CAR T treatment team and patient are vigilant for signs and symptoms of other adverse events too, including serious infection and febrile neutropenia, which are treated accordingly.1 The patient’s blood count and immunoglobulin levels are monitored to identify cytopenias and hypogammaglobulinaemia that may require management.1
Please see the summary of product characteristics for full information on undesirable effects and special warnings and precautions for use of YESCARTA®/TECARTUS®, including guidance on managing CRS and neurological adverse reactions.
Reporting adverse events
Treating hospital and referring hospital
Treating physicians report any suspected adverse reactions to YESCARTA®/TECARTUS® to both the Swedish Medical Products Agency (Läkemedelsverket) and Gilead.1,3 For instructions on reporting, see Contact Us.
Adverse events to related procedures (e.g., apheresis or conditioning therapy) are reported too.4
The European Society for Blood and Marrow Transplantation runs a registry for follow up of patients who received YESCARTA®/TECARTUS®. If the patient has consented, adverse events are reported to the registry also.
This additional monitoring will allow quick identification of new safety information, helping to protect the safety of future patients.
Instructing patients, relatives, and carers on adverse events
Patients (and relatives and carers) play an active role in reducing risks associated with YESCARTA®/TECARTUS® treatment by:1
- contacting their CAR T treatment team or seeking immediate medical attention if they experience any signs or symptoms of CRS or neurological events, such as fever, shortness of breath, extreme tiredness, seizures, and lack of coordination
- carrying their Patient Alert Card at all times and sharing it with treating healthcare professionals
- taking their temperature twice a day for 3–4 weeks
- being alert for signs and symptoms of other adverse events that require immediate medical attention, such as infection, anaemia, and thrombocytopenia
- staying within 2-hours’ journey of the treating hospital for 4 weeks
- not driving or operating heavy or potentially dangerous machines for at least 8 weeks and until any neurological adverse events resolve.
- To meet the criteria to stay within 2 hours of the treatment hospital, patients may need to arrange alternative accommodation such as staying with relatives or at a hotel, residential care, or convalescent care.
Please see the package leaflet for information that should be provided to patients, including a list of signs and symptoms that require immediate medical attention.
References
- Yescarta SmPC
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44.
- European Medicines Agency, Human Medicines Division. Appendix V. EMA/67830/2013, Version 22. 19 March 2021.
- European Commission. Guidelines on good clinical practice specific to advanced therapy medicinal products (text with EEA relevance). C(2019) 7140 Final. Brussels, 10 October 2019.
SE-YES-0025 | 09/2024
Focus on the patient
Common patient questions
Anticipating and addressing the needs and concerns of patients
In the first 7 days after infusion, patients will require personalized information and support depending on the side effects that they experience. General questions that patients may ask include:
- When will I be discharged?
- Can I have visitors?
- What happens if I get a serious side effect?
- How long does it take to recover from CAR T cell infusion?
- When will we know whether my CAR T therapy has worked?
On discharge, patients may ask:
- Is it safe for me to be out of hospital?
- What can I and my family do to keep me as healthy as possible?
- Is there a 24-hour number for advice and medical support?

Supporting patients in managing their risk
Ensuring that patients clearly understand the safety precautions they must take can help to minimize risks and manage anxiety. Ensure that every patient understands and remembers all the safety information they have been given and carries their Patient Alert Card at all times.
Before the patient is discharged, check that he or she will be supported by relatives or carers with any additional care and support needs they have.
Supplying patients with a treatment journal may encourage reporting of side effects.
Please see the package leaflet for information that should be provided to patients, including a list of signs and symptoms that require immediate medical attention.
Information for patients
If you would like patient-friendly information on YESCARTA®/TECARTUS® to help you to explain this treatment to patients, please Contact Us to make a request.
In addition, you may wish to provide to your patients the contact details of trusted patient groups for more information and support on CAR T therapy.*
*Kite does not endorse or make any assurances regarding the accuracy of information on CAR T therapy provided by third party organizations.
