Continued follow up
Treating hospital and referring hospital
- Weekly phone calls from the CAR T treatment team
- First assessment of tumour response
- Handover to the referring physician
- The schedule for follow-up appointments
- Monitoring and reporting adverse events
Medical and clinical responsibility
Weekly phone calls from the CAR T treatment team
Treating hospital: haematologist, oncologist, or nurse
Following discharge, it is good practice for the CAR T treatment team to telephone the patient each week to check on his or her health for at least 4 weeks following infusion. These telephone calls help to spot any signs and symptoms of CRS, neurological toxicity, and other adverse events that the patient may have overlooked.
First assessment of tumour response
The first assessment of tumour response by positron emission tomography (PET) or computed tomography (CT) is usually at 4 weeks.1 This is the first opportunity for the CAR T treatment team to establish the patient’s disease status and to see how well YESCARTA®/TECARTUS® is working.
Handover to the referring physician
CAR T treatment team, referring physician, and administrative personnel
After the first 7 days’ monitoring, the timing of the patient returning to the care of his or her referring physician is decided together by the treating hospital, referring hospital, and patient. In part, timing will depend on the patient’s clinical status, need for CAR T specialist care, and logistics such as travel time.
Although the patient’s routine lymphoma care is likely to transition back to the referring physician at some point, the treating hospital will remain actively involved in follow-up of CAR T treatment. Good communication between the referring and treating hospitals is key to optimizing outcomes and health care experiences for patients.
The schedule of follow-up appointments
CAR T treatment team, referring physician, and administrative personnel
Patients have regular follow-up appointments at the treating hospital or referral hospital to assess their response to YESCARTA®/TECARTUS® treatment and disease status. The treating hospital should see the patient at least once a year, but interim appointments may take place at the referring hospital.
An example schedule is shown in the table; however, follow up is individualized to each patient. If the referring physician is performing the follow up, he or she will share the findings with the treating hospital, and vice versa.
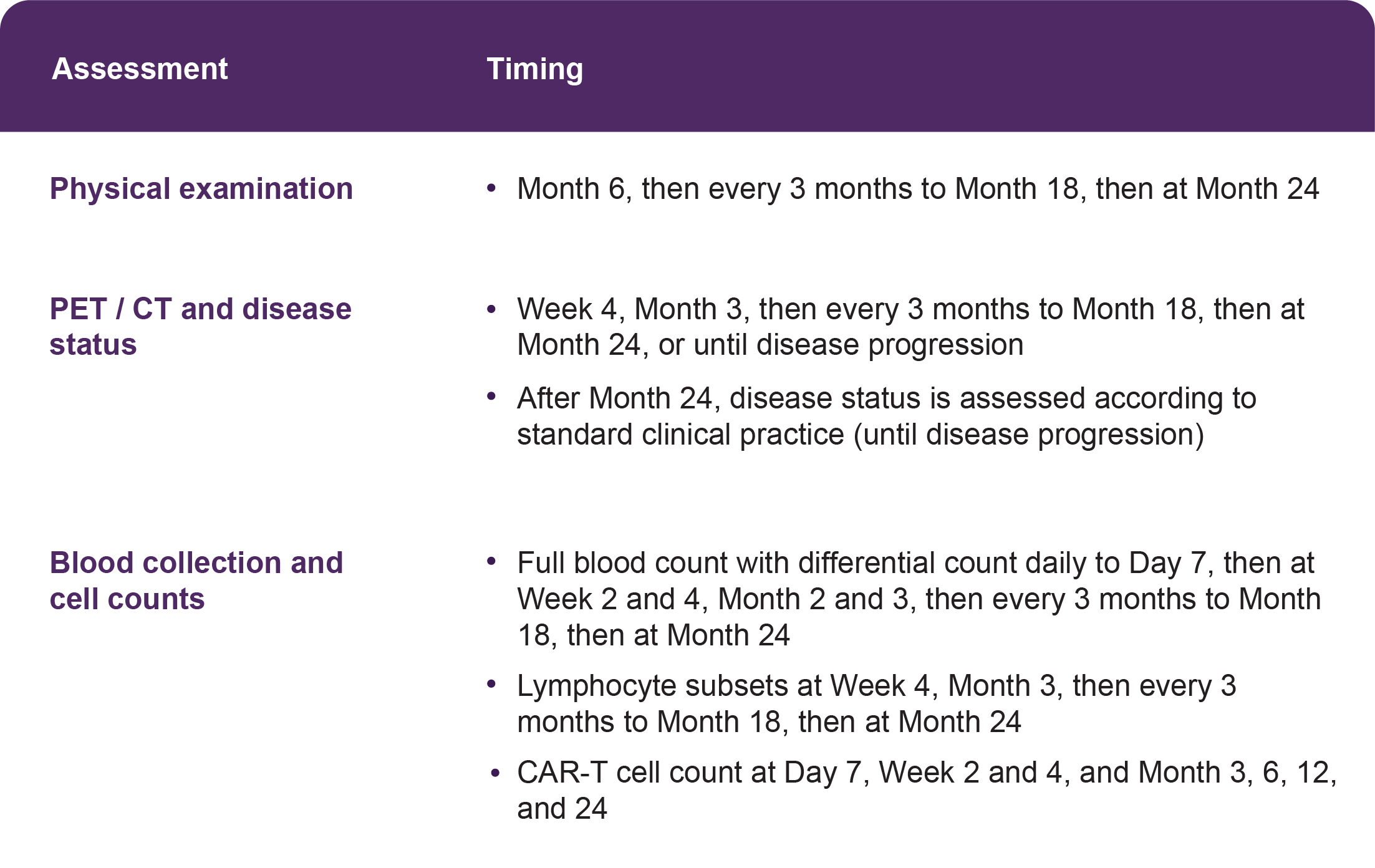
TABLE: Example follow-up schedule after YESCARTA®/TECARTUS® infusion, based on the protocol for: Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44.1 Additional assessments may be required depending on patient health and disease status.
Monitoring and reporting adverse events
CAR T treatment team, referring physician, or other healthcare professionals
After Day 7, the patient’s CAR T treatment team will decide the best approach to monitoring patients for CRS and neurological toxicities.2 The patient will also be monitored for other adverse events including signs of serious infection, cytopenia, and hypogammaglobulinaemia.2
Life-long monitoring for secondary malignancies is also important.2 If secondary malignancies occur, Kite can provide recommendations on sampling to assess whether the malignancy may be linked to CAR T cell therapy.2
Treating physicians report any suspected adverse reactions to YESCARTA®/TECARTUS® to both to both the Swedish Medical Products Agency (Läkemedelsverket) and Gilead.2,3 For instructions on reporting, see Contact Us.
If the patient has consented, adverse events are reported to the European Society for Blood and Marrow Transplantation’s YESCARTA®/TECARTUS® registry too. This additional monitoring will allow quick identification of new safety information, helping to protect the safety of future patients.
Please see the summary of product characteristics for full information on undesirable effects and special warnings and precautions for use of YESCARTA®/TECARTUS®, including guidance on managing CRS and neurological adverse reactions.
References
- Protocol for Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531-44.
- Yescarta SmPC
- European Medicines Agency Human Medicines Division. Appendix V. EMA/67830/2013, Version 22. 19 March 2021.
SE-YES-0026 | 09/2024
Focus on the patient
Common patient questions
Anticipating and addressing the needs and concerns of patients
As patients recover from YESCARTA®/TECARTUS® infusion, their attention is likely to turn to getting back to ‘normal life’ and their remission status. Their questions may include:
- When can I move back home?
- Which hospital will I visit for check-ups?
- Am I in remission and for how long might I stay in remission?
- When can I return to driving / work / hobbies?
- How will we know if my lymphoma comes back?

Supporting patients in managing their risk
Over time, patients may forget some of the safety precautions put in place for YESCARTA®/TECARTUS® treatment. During follow-up telephone calls and appointments, check the patient is still following advice and carrying their Patient Alert Card.
Please see the package leaflet for information that should be provided to patients, including a list of signs and symptoms that require immediate medical attention.
Information for patients
If you would like patient-friendly information on YESCARTA®/TECARTUS® to help you to explain this treatment to patients, please Contact Us to make a request.
In addition, you may wish to provide to your patients the contact details of trusted patient groups for more information and support on CAR T therapy.*
*Kite does not endorse or make any assurances regarding the accuracy of information on CAR T therapy provided by third party organizations.
